Govt gets green light to continue with J&J vaccine rollout
Updated | By Nushera Soodyal
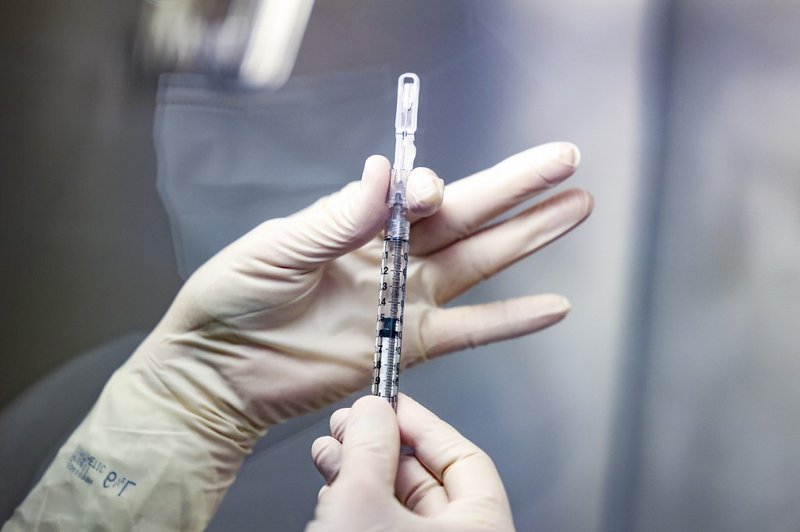
The South African Health Products Regulatory Authority (SAHPRA) has given government the go-ahead to resume the Johnson & Johnson vaccine rollout, but with conditions.
The health products regulator says it's had talks with Janssen Pharmaceutica - as well as the team from the Sisonke trial, which has seen healthcare workers getting the jab.
The use of the J&J vaccine was halted last week after it emerged some recipients in the US had developed blood clots.
SAHPRA's CEO, Boitumelo Semete-Makokotlela says they've reviewed data from the Sisonke study and have decided to lift the pause.
She explains the conditions for the resumption of the rollout.
READ: Over 200 000 people registered for vaccination programme - Pharmacy association
"Screening and monitoring of the participants, particularly those who may present with high risks of blood clotting. There must be safe and immediate management of any participants who may present with any vaccine induced thrombosis.
"Furthermore, the informed consent that the participants sign-off, as well as the participant information share safety sheets, will need to be updated to reflect on any other possible safety concerns."

Show's Stories
-
Temu’s got a local warehouse in SA
Temu has just launched its first warehouse in South Africa! Here’s what ...
East Coast Breakfast 1 day, 22 hours ago -
Green ID book production to be discontinued in SA
The Department of Home Affairs is planning to phase out the green ID boo...
Stacey & J Sbu 2 days, 1 hour ago